This effect was eliminated by silencing tumor cell B AR expression, im plicating tumor cell B AR expression and signaling as an important facilitator of pressure induced tumor angiogen esis in vivo. In vitro studies working with tumor cell lines recommend that catecholamines can market tumor pro gression by a B AR driven proangiogenic pathway. This stimulation of VEGF expression by B adrenergic signaling is proportional to B AR expression, dose dependent and inhibited by B AR antagonists. There is certainly evidence that expression of VEGF in endothelial cells may also be controlled by adrenergic stimulation. as demonstrated in numerous in vitro and in vivo models, B AR agonists, such as epinephrine, norepinephrine and ISO, can induce the expression of VEGF. Conversely, B AR antagonists cause a diminished expres sion of VEGF and inhibit cell proliferation and angio genesis.
While in the existing review, ISO improved the expression degree of VEGF A in HemECs inside a B AR and ERK dependent method. These findings are consistent with earlier studies in which B AR stimulation resulted in the more than expression of VEGF A by means of the B AR and ERK signaling cascade. We also uncovered the more helpful hints ISO stimulated activation of ERK and subsequent proliferation of HemECs expected VEGFR two exercise. Scientific studies have shown that cultured HemECs share a phenotype of constitutively active VEGFR two signaling, which could possibly render the cells even more sensitive to autocrine or paracrine stimulation of VEGF A. The VEGFR 2 intracellular signaling pathway in HemECs was not entirely explored, but success from your in vitro VEGF A stimulation of various types of endothe lial cells indicated that VEGFR 2 signaling is dependent for the downstream results of ERK. Though activation of VEGFR 2 and B ARs continues to be implicated while in the promotion of cell proliferation, the connection in between these two receptor systems is poorly understood.
Here, we produce the very first evidence that the VEGFR 2 mediated phosphorylation of ERK is upregulated on B AR activation to mediate proliferation of HemECs. These findings, together with the observation Cilostazol the ISO induced phosphorylation of VEGFR 2 can be inhibited by ICI, show the transactivation of VEGFR 2 may perhaps act as an effector pathway to mediate the mitogenic results in the 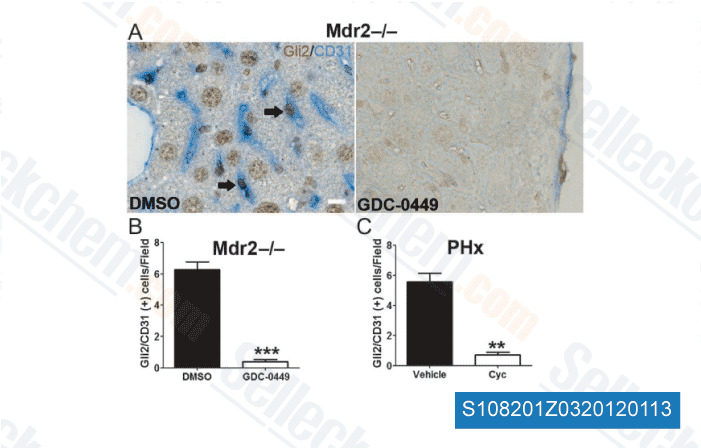 B ARs. In conclusion, we demonstrated that activation with the B ARs resulted in greater HemEC proliferation and upregulation on the ERK signaling cascade. VEGFR two mediated ERK signaling was also upregulated upon B AR activation to mediate proliferation of HemECs. These findings not only give a pharmacological basis for that therapeutic use of B AR antagonists while in the treatment of IH but additionally unveil a functional connection in between the B ARs and VEGFR two in HemECs.
B ARs. In conclusion, we demonstrated that activation with the B ARs resulted in greater HemEC proliferation and upregulation on the ERK signaling cascade. VEGFR two mediated ERK signaling was also upregulated upon B AR activation to mediate proliferation of HemECs. These findings not only give a pharmacological basis for that therapeutic use of B AR antagonists while in the treatment of IH but additionally unveil a functional connection in between the B ARs and VEGFR two in HemECs.
Peptide Solubility
The prohormones are then packaged into membrane-bound secretory vesicles.
